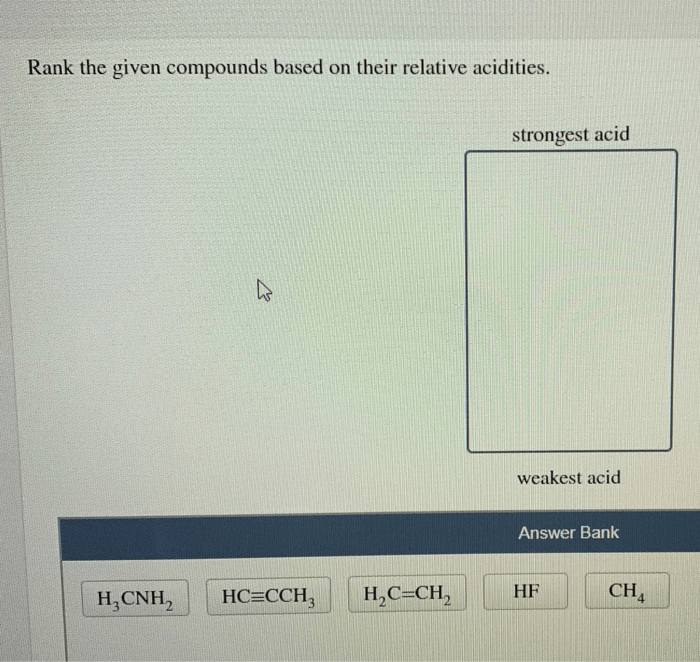
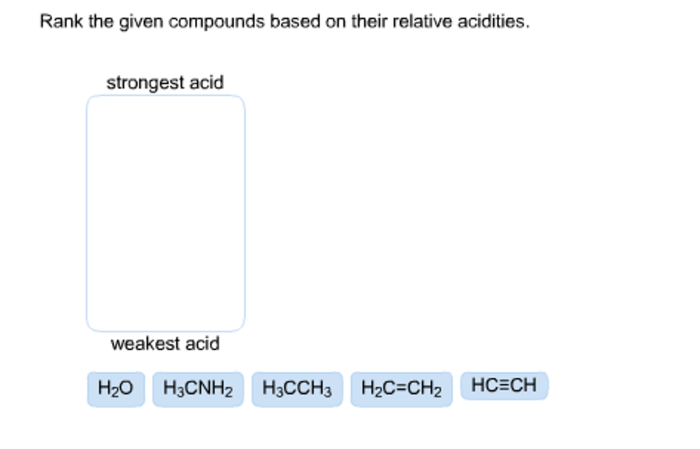
Rank the given compounds based on their relative acidities, a topic of paramount importance in chemistry, unveils the intriguing relationship between molecular structure and acidity. This concept, measured through pKa values, forms the cornerstone of understanding the behavior of acids and their applications in diverse fields.
By delving into the factors influencing acid strength, such as electronegativity and resonance, we gain insights into the variations in acidity among different compounds. This knowledge empowers us to predict and control the reactivity of acids, enabling advancements in chemical synthesis, drug development, and various other disciplines.
Understanding Acid Strength: Rank The Given Compounds Based On Their Relative Acidities
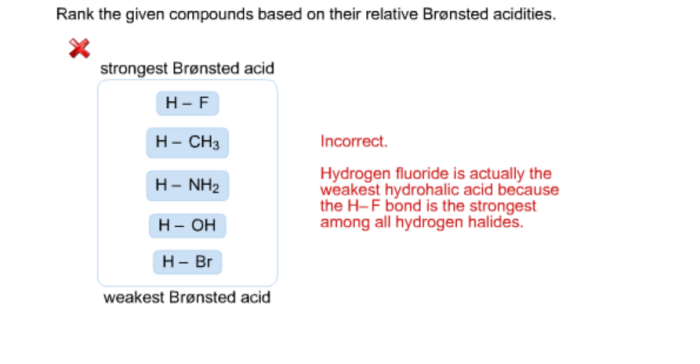
Acidity is a measure of a substance’s ability to donate protons (H+ ions). The strength of an acid is quantified using the pKa value, which represents the negative logarithm of the acid dissociation constant (Ka). A lower pKa value indicates a stronger acid.
Factors that influence acid strength include electronegativity and resonance. Electronegative atoms attract electrons more strongly, making it easier for them to release protons. Resonance stabilizes the conjugate base of the acid, making it more difficult for the acid to donate protons.
Ranking Compounds by Acidity
| Compound | pKa |
|---|---|
| Hydrochloric acid (HCl) | |
| Sulfuric acid (H2SO4) | |
| Nitric acid (HNO3) | |
| Acetic acid (CH3COOH) | |
| Phenol (C6H5OH) |
Using the pKa values, we can rank the compounds in order of decreasing acidity:
- Hydrochloric acid
- Sulfuric acid
- Nitric acid
- Acetic acid
- Phenol
Comparing Acidic Properties
Hydrochloric acid is the strongest acid among the given compounds, with the lowest pKa value of -7. Phenol is the weakest acid, with the highest pKa value of 10.
The difference in acidity between the compounds is due to the stability of their conjugate bases. The conjugate base of a strong acid is more stable than the conjugate base of a weak acid. This stability difference is reflected in the pKa values.
Structural Features and Acidity, Rank the given compounds based on their relative acidities
Structural features can also affect the acidity of a compound. Functional groups that can stabilize the conjugate base make the acid weaker. For example, the carboxylic acid group (-COOH) in acetic acid stabilizes the conjugate base through resonance, making acetic acid a weaker acid than hydrochloric acid.
Molecular geometry can also influence acidity. Compounds with tetrahedral geometry, such as sulfuric acid, are typically stronger acids than compounds with trigonal planar geometry, such as nitric acid.
Applications of Acidity Ranking
Ranking acids based on their relative acidities is important in many fields, including chemistry, medicine, and materials science.
- In chemistry, acidity ranking is used to predict the products of reactions and to design new catalysts.
- In medicine, acidity ranking is used to develop drugs and to understand the effects of acids on the human body.
- In materials science, acidity ranking is used to design materials with specific properties, such as corrosion resistance and biocompatibility.
FAQ Section
What is the significance of ranking acids based on their relative acidities?
Ranking acids based on their relative acidities allows us to predict their reactivity and behavior in chemical reactions. It helps us understand the strength of acids and their ability to donate protons, which is crucial in various chemical and biological processes.
How are pKa values used to rank acids?
pKa values are logarithmic measures of acid strength. The lower the pKa value, the stronger the acid. By comparing the pKa values of different acids, we can determine their relative acidities and predict their behavior in reactions.
What factors influence the acidity of a compound?
The acidity of a compound is influenced by several factors, including the electronegativity of the atom bonded to the acidic hydrogen, the resonance stabilization of the conjugate base, and the inductive effects of neighboring groups.