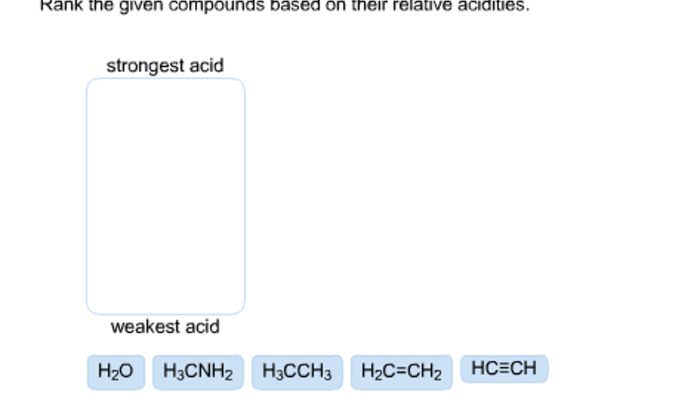
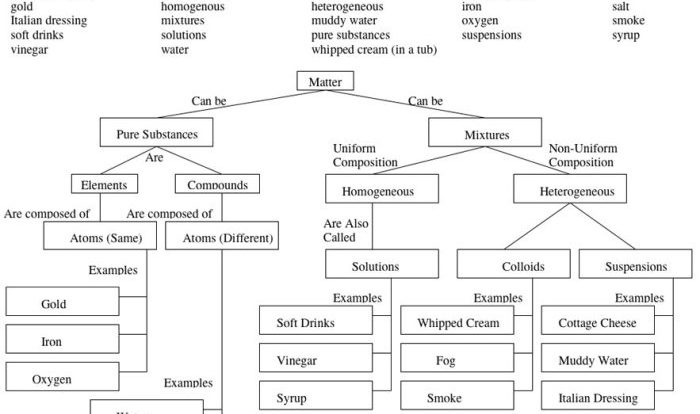
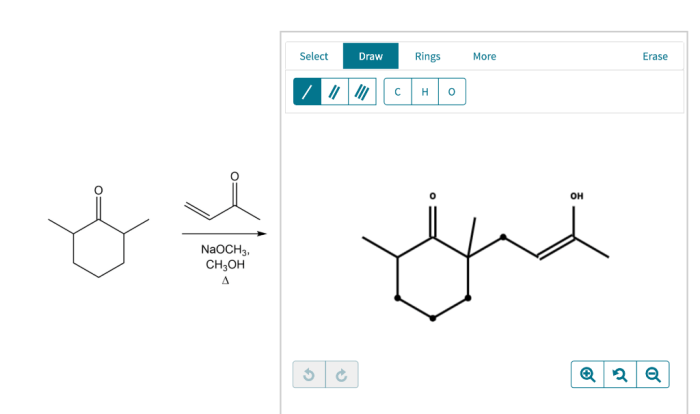
Chemical reaction systems unit test – Embark on a captivating journey into the realm of chemical reaction systems with our comprehensive unit test. Dive deep into the fundamental principles that govern these dynamic systems, unraveling the intricacies of reactants, products, equilibrium, and kinetics.
Our exploration extends to the diverse types of chemical reactions, from synthesis to combustion, revealing their unique characteristics and providing real-world examples. We’ll investigate the factors that influence reaction rates and delve into the concept of chemical equilibrium, understanding its profound impact on reaction dynamics.
Key Concepts and Definitions
Chemical reaction systems are the foundation of chemistry, involving the study of how substances change into different substances. Understanding the fundamental principles governing these systems is crucial.
Reactants are the initial substances that undergo a chemical change, while products are the new substances formed. Equilibrium is a state of balance where the concentrations of reactants and products remain constant over time. Kinetics describes the rate at which chemical reactions occur, influenced by factors like temperature, concentration, and the presence of catalysts.
Reactants
- Substances present at the beginning of a chemical reaction.
- Undergo chemical change to form products.
- Examples: Hydrogen (H2) and Oxygen (O2) in the combustion reaction.
Products
- Substances formed as a result of a chemical reaction.
- Created from the rearrangement of atoms in the reactants.
- Examples: Water (H2O) and Carbon Dioxide (CO2) in the combustion reaction.
Equilibrium
- A state of balance in a chemical reaction.
- Reactants and products are continuously interconverting, but their concentrations remain constant.
- Examples: The Haber process for ammonia production.
Kinetics
- The study of the rate of chemical reactions.
- Factors influencing reaction rates include temperature, concentration, and catalysts.
- Examples: The use of enzymes to accelerate biochemical reactions.
Types of Chemical Reactions
Chemical reactions are processes that involve the transformation of one set of chemical substances to another. These reactions can be classified into various types based on the nature of the changes that occur during the reaction. Here are some common types of chemical reactions:
There are several types of chemical reactions, each with its own characteristics and applications. Understanding the different types of reactions is essential for comprehending chemical processes and predicting the outcome of chemical interactions.
Synthesis Reactions
Synthesis reactions are reactions in which two or more substances combine to form a single product. These reactions are often represented using the general equation:
A + B → AB
where A and B are the reactants and AB is the product.
Examples of synthesis reactions include:
- The formation of water from hydrogen and oxygen: 2H 2+ O 2→ 2H 2O
- The formation of sodium chloride from sodium and chlorine: 2Na + Cl 2→ 2NaCl
- The formation of carbon dioxide from carbon and oxygen: C + O 2→ CO 2
Reaction Rates and Equilibrium: Chemical Reaction Systems Unit Test
Reaction rates and equilibrium are crucial concepts in understanding chemical reactions. Factors like temperature, concentration, and surface area influence reaction rates, while equilibrium represents a state where forward and reverse reactions occur at equal rates.
Factors Affecting Reaction Rates
- Temperature:Higher temperatures increase the kinetic energy of molecules, leading to more frequent and energetic collisions, resulting in faster reaction rates.
- Concentration:Higher concentrations of reactants increase the likelihood of collisions, speeding up reaction rates.
- Surface Area:For reactions involving solids, increasing the surface area exposes more reactive sites, facilitating faster reactions.
Chemical Equilibrium
Chemical equilibrium is a dynamic state where the forward and reverse reactions occur at equal rates. At equilibrium, the concentrations of reactants and products remain constant over time.
Equilibrium is influenced by several factors, including:
- Temperature:Changing temperature shifts the equilibrium position towards the endothermic or exothermic reaction.
- Concentration:Adding or removing reactants or products can shift the equilibrium position to favor the corresponding reaction.
Balancing Chemical Equations
Chemical equations represent the transformation of reactants into products. Balancing these equations ensures that the number of atoms of each element is the same on both sides of the equation, adhering to the law of conservation of mass.
To balance chemical equations, follow these steps:
- Write the unbalanced equation, ensuring all reactants and products are included.
- Start by balancing the most complex molecule, typically the one with the most atoms.
- Balance one element at a time, adjusting the stoichiometric coefficients in front of each chemical formula.
- Do not change the subscripts within the chemical formulas, as this would alter the identity of the compound.
- Continue balancing until all elements are balanced.
Example
Balance the following equation:
“`
C2H6 + 7O2 → 4CO2 + 6H2O
“`
Starting with carbon, we have 4 atoms on the product side and 4 atoms on the reactant side, so it is already balanced.
For hydrogen, we have 12 atoms on the product side and 12 atoms on the reactant side, so it is also balanced.
For oxygen, we have 14 atoms on the product side and 14 atoms on the reactant side, so it is balanced as well.
Therefore, the equation is already balanced.
Stoichiometry
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It is essential for understanding the behavior of chemical systems and for making predictions about the outcome of reactions.
Stoichiometry can be used to calculate the amounts of reactants and products involved in a reaction. This information can be used to design experiments, optimize reaction conditions, and predict the products of a reaction.
Balanced Chemical Equations
Balanced chemical equations are essential for stoichiometry. A balanced chemical equation shows the number of atoms of each element on both sides of the equation. This ensures that the law of conservation of mass is obeyed.
To balance a chemical equation, coefficients are added to the reactants and products. Coefficients are numbers that tell us how many molecules of each reactant and product are involved in the reaction. The coefficients are adjusted until the number of atoms of each element is the same on both sides of the equation.
Thermodynamics and Chemical Reactions
Thermodynamics plays a pivotal role in chemical reaction systems, providing insights into the energy changes that accompany chemical reactions. It involves the study of the relationships between heat, work, and energy in chemical systems.Three key thermodynamic concepts are crucial in understanding chemical reactions: enthalpy, entropy, and free energy.
Enthalpy (H) measures the total thermal energy of a system, including both internal energy and the energy associated with volume changes. Entropy (S) quantifies the disorder or randomness within a system. Free energy (G) combines enthalpy and entropy, providing a measure of the maximum useful work that can be obtained from a system at constant temperature and pressure.
Enthalpy and Chemical Reactions
Enthalpy changes (ΔH) indicate the heat absorbed or released during a chemical reaction. Exothermic reactions release heat (ΔH < 0), while endothermic reactions absorb heat (ΔH > 0). The magnitude of ΔH indicates the amount of heat involved in the reaction.
Entropy and Chemical Reactions
Entropy changes (ΔS) measure the change in disorder or randomness during a reaction. Reactions that increase disorder (e.g., gas formation) have positive ΔS values, while reactions that decrease disorder (e.g., precipitation) have negative ΔS values.
Free Energy and Chemical Reactions, Chemical reaction systems unit test
Free energy changes (ΔG) combine enthalpy and entropy to determine the spontaneity of a reaction. Spontaneous reactions occur with a decrease in free energy (ΔG < 0), indicating that the reaction proceeds without external input. Non-spontaneous reactions have a positive ΔG, requiring external energy to drive the reaction. Understanding these thermodynamic concepts enables the prediction of reaction spontaneity, energy requirements, and the direction of chemical reactions.
Applications of Chemical Reaction Systems
Chemical reaction systems have a wide range of practical applications across various fields, including industrial processes, medicine, and environmental science.
These systems are used to create new materials, synthesize drugs, and monitor and control environmental conditions.
Industrial Processes
- Production of Chemicals:Chemical reaction systems are used to produce a vast array of chemicals, including plastics, fertilizers, and pharmaceuticals. For example, the Haber process uses a chemical reaction between nitrogen and hydrogen to produce ammonia, which is essential for fertilizer production.
- Refining of Petroleum:Chemical reaction systems are employed in oil refineries to convert crude oil into gasoline, diesel fuel, and other products. These systems involve a series of chemical reactions, such as cracking and reforming, to break down and rearrange the molecules in the crude oil.
- Manufacturing of Materials:Chemical reaction systems are used to produce a variety of materials, such as ceramics, glass, and metals. For example, the production of cement involves a chemical reaction between limestone and clay to form calcium silicate, the main component of cement.
Common Queries
What is the significance of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side matches the number of atoms of the same element on the products’ side, adhering to the law of conservation of mass.
How does temperature affect reaction rates?
Increasing temperature generally increases reaction rates because it provides more energy for the reacting molecules to overcome the activation energy barrier.
What is the role of catalysts in chemical reactions?
Catalysts are substances that increase the rate of a reaction without being consumed. They provide an alternative reaction pathway with a lower activation energy, facilitating the reaction.